Haydari MN, Perron MC, Laprise S, Roy O, Cameron JD, Proulx S, Brunette I. A short-term in vivo experimental model for Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2012 Sep 19;53(10):6343-54.
Scientific impact: Fuchs endothelial dystrophy is an ocular degenerative disease causing corneal thickening and edema and loss of transparency. This results in light sensitivity and painful loss of vision. Corneal graft is the last resort to treat this disease which is responsible of more than a quarter of the 50 000 yearly corneal grafts in Canada and the US. As ocular tissue banks are limited, novel sources of healthy tissue are necessary. This paper demonstrates for the first time that it is possible to grow endothelial cells from Fuchs dystrophia patients without genetic engineering. These cells have been used to develop novel in vitro and in vivo models to study this disease. They have also made it possible to reconstruct functional corneal endothelium once transplanted in animals. These results pave the way for a new therapeutic avenue in which a patient could be treated with his own cells.
Network contribution: The VHRN provided human endothelial cells with Fuchs endothelial dystrophia (ocular tissue bank) to the many teams responsible for this multidisciplinary collaboration project.
* * *
Original Abstract
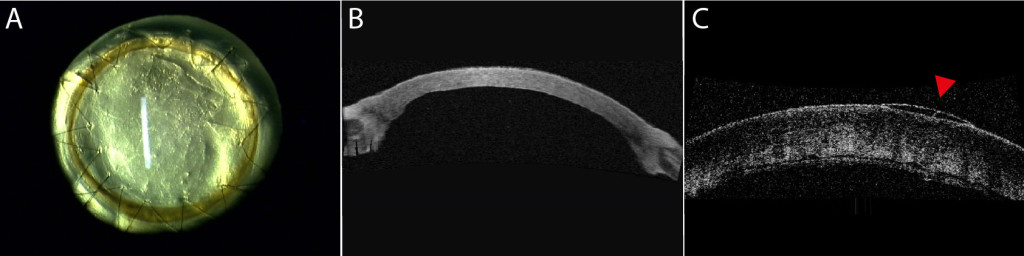
Purpose: We evaluated the in vivo functionality of a corneal endothelium tissue-engineered using corneal endothelial cells from human patients with Fuchs endothelial corneal dystrophy (FECD). Methods: A total of 15 healthy cats underwent full-thickness corneal transplantation. All transplants were of xenogeneic human origin and all grafts but two were tissue-engineered. In seven animals the graft corneal endothelium was tissue-engineered using cultured corneal endothelial cells from humans with FECD (TE-FECD). Two control animals were grafted with an endothelium engineered using cultured endothelial cells from normal eye bank corneas (TE-normal). Two controls received a native full-thickness corneal transplant, and four other controls were grafted with the stromal carrier only (without endothelial cells). Outcome parameters included graft transparency (0, opaque to 4, clear), pachymetry, optical coherence tomography, endothelial cell morphometry, transmission electron microscopy (TEM), and immunostaining of function-related proteins. Results: Seven days after transplantation, 6 of 7 TE-FECD grafts, all TE-normal grafts, and all normal native grafts were clear (transparency score >3), while all carriers-only grafts were opaque (score <1). The mean pachymetry was 772 ± 102 μm for TE-FECD, 524 ± 11 μm for TE-normal, 555 ± 48 for normal native, and 1188 ± 223 μm for carriers only. TEM showed subendothelial loose fibrillar material deposition in all TE-FECD grafts. The TE endothelium expressed Na(+)-K(+)/ATPase and Na(+)/HCO3(-). Conclusions: Restoration of transparency and corneal thickness demonstrated that the TE-FECD grafts were functional in vivo. This novel FECD seven-day living model suggests a potential role for tissue engineering leading to FECD cell rehabilitation.
Modèle expérimental in-vivo de dystrophie de Fuchs (A, B) et son contrôle oedmacié, sans endothlium (C).