Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honoré JC, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beauséjour C, Lachapelle P, Smith LE, Chemtob S, Sapieha P. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011 Jun 2;117(22):6024-35.
Scientific impact: Diabetic retinopathies are ocular disease and an important cause of blindness worldwide. They have many origins (diabetes, oxygen at birth), and are generally considered as a vascular disorder caused by a deregulation of retinal oxygenation. Even though it is extensively studied, the development process of this disease is still not fully understood. This article demonstrates that during prolonged ischemia, retinal ganglion cells de produce a vaso-repulsive molecule, Semaphorin 3A, which blocks vascular regeneration in the most hypoxic regions of the retina. This new knowledge allows us to understand a key aspect of disease progression and make Semaphorin 3A an ideal target for the treatment of this disease.
Network contribution: This work was done as collaboration between many members of the VHRN and was partly supported by VHRN grants (student bursary of Nicholas Sitaras and François Binet).
* * *
Original Abstract
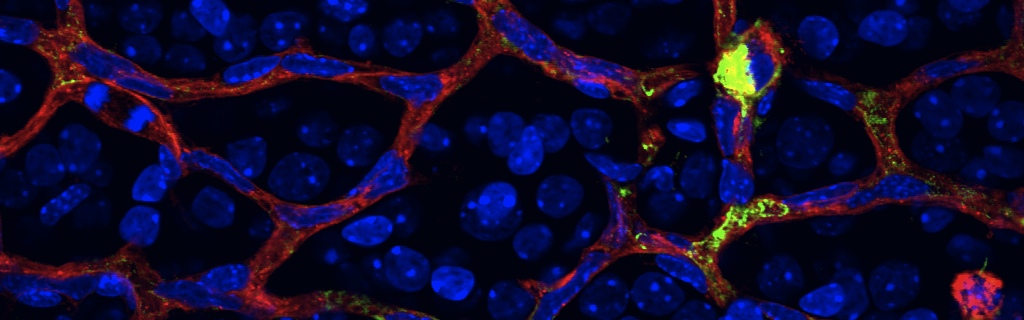
The failure of blood vessels to revascularize ischemic neural tissue represents a significant challenge for vascular biology. Examples include proliferative retinopathies (PRs) such as retinopathy of prematurity and proliferative diabetic retinopathy, which are the leading causes of blindness in children and working-age adults. PRs are characterized by initial microvascular degeneration, followed by a compensatory albeit pathologic hypervascularization mounted by the hypoxic retina attempting to reinstate metabolic equilibrium. Paradoxically, this secondary revascularization fails to grow into the most ischemic regions of the retina. Instead, the new vessels are misdirected toward the vitreous, suggesting that vasorepulsive forces operate in the avascular hypoxic retina. In the present study, we demonstrate that the neuronal guidance cue semaphorin 3A (Sema3A) is secreted by hypoxic neurons in the avascular retina in response to the proinflammatory cytokine IL-1β. Sema3A contributes to vascular decay and later forms a chemical barrier that repels neo-vessels toward the vitreous. Conversely, silencing Sema3A expression enhances normal vascular regeneration within the ischemic retina, thereby diminishing aberrant neovascularization and preserving neuroretinal function. Overcoming the chemical barrier (Sema3A) released by ischemic neurons accelerates the vascular regeneration of neural tissues, which restores metabolic supply and improves retinal function. Our findings may be applicable to other neurovascular ischemic conditions such as stroke.
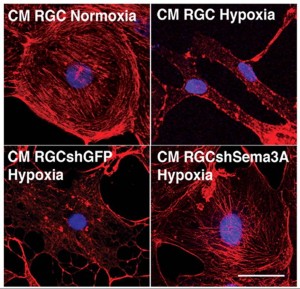
Hypoxia destroys the actin network of endothelial cells. This reaction is blocked when Semaphorin3a production is inhibited