Binet F, Mawambo G, Sitaras N, Tetreault N, Lapalme E, Favret S, Cerani A, Leboeuf D, Tremblay S, Rezende F, Juan AM, Stahl A, Joyal JS, Milot E, Kaufman RJ, Guimond M, Kennedy TE, Sapieha P. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1α degradation of netrin-1. Cell Metab. 2013 Mar 5;17(3):353-71.
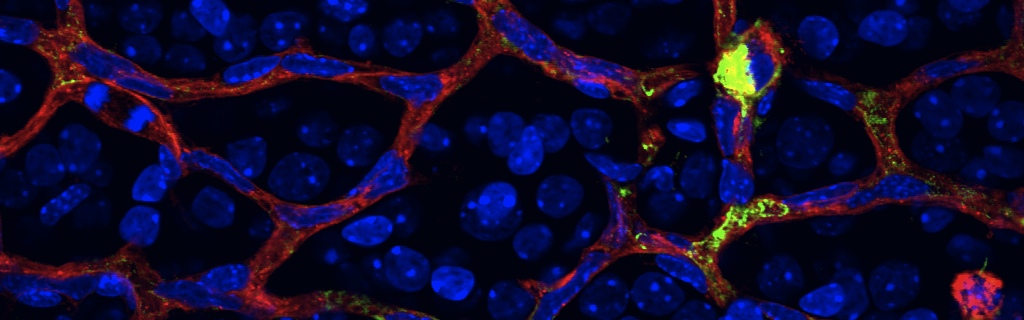
Scientific impact: This study identifies a fundamental mechanism by which neurons communicate with the immune system to repair damaged blood vessels. More specifically, this article demonstrates that the nerve cells of the eye responsible for relaying visual information from the eye to the brain (retinal ganglion neurons) can produce a molecule called netrin that has previously documented roles as a neuro-vascular guidance cue. Netrin is made by healthy or moderately injured neurons and prompts immune cells to release factors that help regenerate functional blood vessels. In diseases such as diabetic retinopathy, netrin-producing neurons are deprived of oxygen/nutrients for a prolonged period and a cellular program is activated that stops production of netrin. Consequently, the eye’s endogenous repair programs are compromised. The findings of this study can be harnessed towards the design of therapeutic strategies to stunt the progression of diseases such as diabetic retinopathy and ultimately to regenerate functional vessels into hypoxic nervous tissue.
Network contribution: The VHRN has given a bursary to the principal author of this study, which contributed to the realization of this project.
* * *
Original Abstract
In stroke and proliferative retinopathy, despite hypoxia driven angiogenesis, delayed revascularization of ischemic tissue aggravates the loss of neuronal function. What hinders vascular regrowth in the ischemic central nervous system remains largely unknown. Using the ischemic retina as a model of neurovascular interaction in the CNS, we provide evidence that the failure of reparative angiogenesis is temporally and spatially associated with endoplasmic reticulum (ER) stress. The canonical ER stress pathways of protein kinase RNA-like ER kinase (PERK) and inositol-requiring enzyme-1α (IRE1α) are activated within hypoxic/ischemic retinal ganglion neurons, initiating a cascade that results in angiostatic signals. Our findings demonstrate that the endoribonuclease IRE1α degrades the classical guidance cue netrin-1. This neuron-derived cue triggers a critical reparative-angiogenic switch in neural macrophage/microglial cells. Degradation of netrin-1, by persistent neuronal ER stress, thereby hinders vascular regeneration. These data identify a neuronal-immune mechanism that directly regulates reparative angiogenesis.
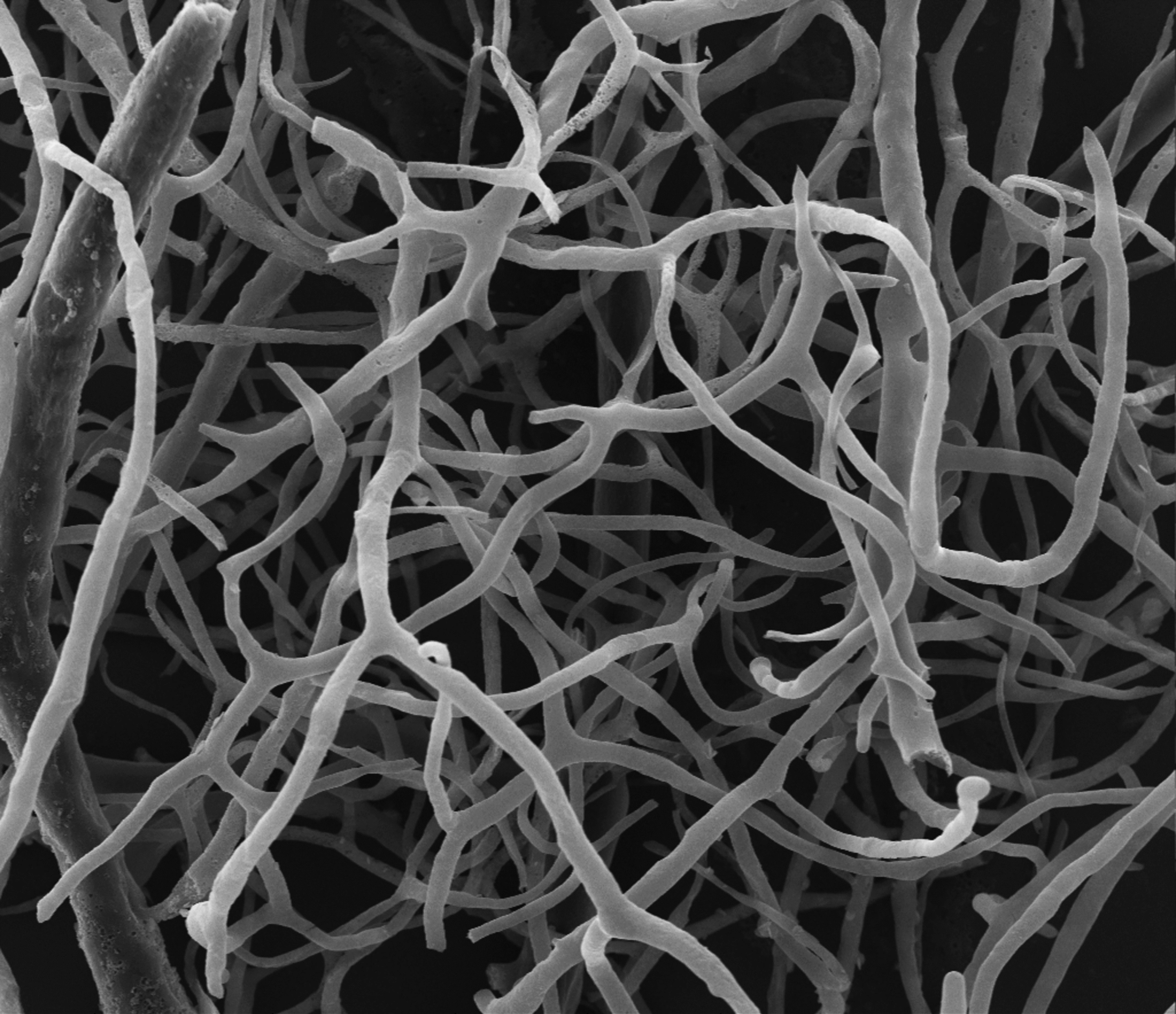
Electron microscopy image of a mouse retinal vasculature