Manuguerra-Gagné R. Boulos PR, Ammar A, Leblond FA, Krosl G, Pichette V, Lesk MR, Roy DC. Stem Cells. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. 2013 Jun;31(6):1136-48
Scientific impact: Glaucoma is an ocular degenerative disease that leads to a loss of vision caused by a rise in ocular pressure. The origin of its development is the loss of function of the trabecular meshwork, responsible of aqueous humor outflow. For degenerative diseases like glaucoma, stem cell research is an extremely promising field of research. Amongst them, mesenchymal stem cells (MSC) are capable of accelerating wound healing. This article demonstrates that MSC are capable of activating local progenitor cells that will migrate to the trabeculum and replace damaged cells. These results could lead to clinical treatments that could considerably improve the quality of life of glaucoma patients.
Network contribution: The VHRN has given a bursary to the principal author of this study, which contributed to the realization of this project.
* * *
Original Abstract
Among bone marrow cells, hematopoietic and mesenchymal components can contribute to repair damaged organs. Such cells are usually used in acute diseases but few options are available for the treatment of chronic disorders. In this study, we have used a laser-induced model of open angle glaucoma (OAG) to evaluate the potential of bone marrow cell populations and the mechanisms involved in tissue repair. In addition, we investigated laser-induced tissue remodeling as a method of targeting effector cells into damaged tissues. We demonstrate that among bone marrow cells, mesenchymal stem cells (MSC) induce trabecular meshwork regeneration. MSC injection into the ocular anterior chamber leads to far more efficient decrease in intraocular pressure (IOP) (p < .001) and healing than hematopoietic cells. This robust effect was attributable to paracrine factors from stressed MSC, as injection of conditioned medium from MSC exposed to low but not to normal oxygen levels resulted in an immediate decrease in IOP. Moreover, MSC and their secreted factors induced reactivation of a progenitor cell pool found in the ciliary body and increased cellular proliferation. Proliferating cells were observed within the chamber angle for at least 1 month. Laser-induced remodeling was able to target MSC to damaged areas with ensuing specific increases in ocular progenitor cells. Thus, our results identify MSC and their secretum as crucial mediators of tissue repair in OAG through reactivation of local neural progenitors. In addition, laser treatment could represent an appealing strategy to promote MSC-mediated progenitor cell recruitment and tissue repair in chronic diseases.
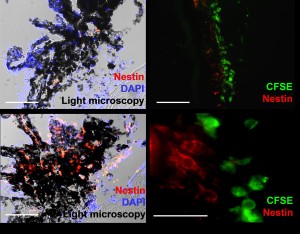
This image shows that mesenchymal cells (green) lead to the recruitment of progenitor cells (red) in a damaged eye (right and bottom left). Endogenous cells are not present in the absence of MSC (top left).