Joyal JS, Nim S, Zhu T, Sitaras N, Rivera JC, Shao Z, Sapieha P, Hamel D, Sanchez M, Zaniolo K, St-Louis M, Ouellette J, Montoya-Zavala M, Zabeida A, Picard E, Hardy P, Bhosle V, Varma DR, Gobeil F Jr, Beauséjour C, Boileau C, Klein W, Hollenberg M, Ribeiro-da-Silva A, Andelfinger G, Chemtob S. Subcellular localization of coagulation factor II receptor-like 1 in neurons governs angiogenesis. Nat Med. 2014 Oct;20(10):1165-73.
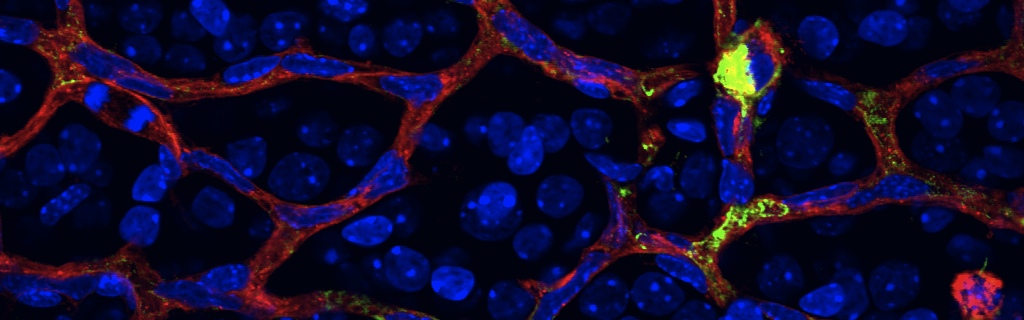
Scientific impact: G protein-coupled receptors (GPCR) have traditionally been shown to function at their plasma membrane location on cells. The actual study published in Nature Medicine, shows for the first time the innovated concept on the migration of a GPCR (namely coagulation factor II receptor-like 1) from the plasma membrane to the nucleus of retinal neurons, which in turn regulates specific angiogenic genes (distinct from those activated by the plasma membrane receptor), and consequently governs in vivo retinal angiogenesis. Since many drugs act on GPCR irrespective of their site of action in the cell, the finding unveils the need to design drugs formulated to specifically target nuclear GPCR, to prevent the abnormal growth of retinal blood vessels and ensued blindness in ischemic retinopathies, such as of diabetes and prematurity.
Network contribution: The RRSV has enabled to consolidate collaborations between members of the RRSV (eg. Chemtob, Hardy, Sapieha), as well as with members of other networks of the FRQS (eg. Gobeil, Beauséjour, Andelfinger), and to support trainees in vision sciences (eg. Shao, Zaniolo, Picard, Hamel).
* * *
Original Abstract
Neurons have an important role in retinal vascular development. Here we show that the G protein–coupled receptor (GPCR) coagulation factor II receptor-like 1 (F2rl1, previously known as Par2) is abundant in retinal ganglion cells and is associated with new blood vessel formation during retinal development and in ischemic retinopathy. After stimulation, F2rl1 in retinal ganglion cells translocates from the plasma membrane to the cell nucleus using a microtubule-dependent shuttle that requires sorting nexin 11 (Snx11). At the nucleus, F2rl1 facilitates recruitment of the transcription factor Sp1 to trigger Vegfa expression and, in turn, neovascularization. In contrast, classical plasma membrane activation of F2rl1 leads to the expression of distinct genes, including Ang1, that are involved in vessel maturation. Mutant versions of F2rl1 that prevent nuclear relocalization but not plasma membrane activation interfere with Vegfa but not Ang1 expression. Complementary angiogenic factors are therefore regulated by the subcellular localization of a receptor (F2rl1) that governs angiogenesis. These findings may have implications for the selectivity of drug actions based on the subcellular distribution of their targets.
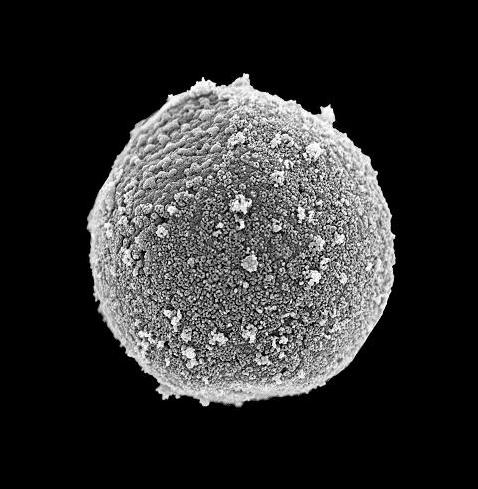
Purity of isolated nuclei confirmed by scanning electron microscopy