Agustin Cerani, Nicolas Tetreault, Catherine Menard, Eric Lapalme, Chintan Patel, Nicholas Sitaras, Felix Beaudoin, Dominique Leboeuf, Vincent De Guire, François Binet, Agnieszka Dejda, Flavio A. Rezende, Khalil Miloudi, Przemyslaw Sapieha. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell Metab. 2013 Oct 1;18(4):505-18.
→ Download the article
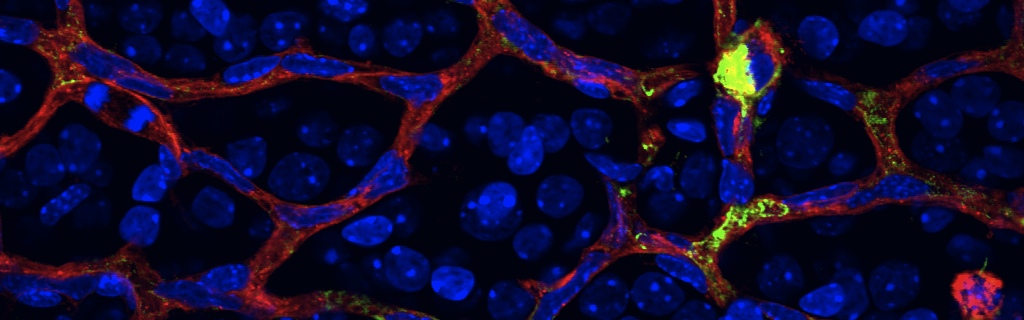
Scientific impact: This study allows the identification of a novel pathological mechanism that causes diabetic macular edema and provides an experimental therapeutic avenue for the treatment of this major sight threatening complication of diabetes. The authors demonstrate in both human patients and animal models that a molecule called Semaphorin3A is robustly induced in the early stages of diabetes and mediates the breakdown of the inner blood retinal barrier. Semaphorin3A is typically recognized for its role in guiding developing neurons and blood vessels during embryogenesis and typically significantly less present in adult tissue. Using a series of experimental approaches including a highly clinically relevant neutralization strategy, the authors demonstrate that inhibiting Semaphorin3A in diabetic eyes may represent an attractive therapeutic alternative to counter sight-threatening vascular permeability. Because Semaphorin3A is typically not present in healthy adult tissue, it is a relatively safe therapeutic target and its pharmacological targeting should not present any signification side-effects. Hence, this study provides a novel experimental intervention to slow this major cause of blindness.
Network contribution: The VHRN has given a bursary to the principal author of this study, which contributed to the realization of this project.
* * *
Original abstract
The deterioration of the inner blood-retinal barrier and consequent macular edema is a cardinal manifestation of diabetic retinopathy (DR) and the clinical feature most closely associated with loss of sight. We provide evidence from both human and animal studies for the critical role of the classical neuronal guidance cue, semaphorin 3A, in instigating pathological vascular permeability in diabetic retinas via its cognate receptor neuropilin-1. We reveal that semaphorin 3A is induced in early hyperglycemic phases of diabetes within the neuronal retina and precipitates initial breakdown of endothelial barrier function. We demonstrate, by a series of orthogonal approaches, that neutralization of semaphorin 3A efficiently prevents diabetes-induced retinal vascular leakage in a stage of the disease when vascular endothelial growth factor neutralization is inefficient. These observations were corroborated in Tg(Cre-Esr1)/Nrp1(flox/flox) conditional knockout mice. Our findings identify a therapeutic target for macular edema and provide further evidence for neurovascular crosstalk in the pathogenesis of DR.
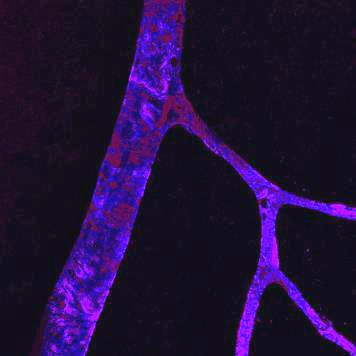
Nascent retinal vessels with microglia interacting with tip cells at the leading edge.